Hard water minerals are like magnets - they stick to everything. Vulcan uses gentle frequency pulses to flip their polarity, making them repel each other instead of clinging to your pipes. They simply wash away, leaving your plumbing scale-free.

A very detailed scientific explanation of the science behind physical water treatment and principal insights into how the Vulcan mineral descaling technology works
1. Introduction
The physical water treatment has been used and discussed for the last two decades. During this time, it has proven its effectiveness that on the other hand is still questioned and denied. Why is that? If we follow the discussions, we can find various reasons that however are not going to be discussed here. It rather seems necessary to examine the physical foundations that can explain the mode of action of these processes and so to free them from the reproach of fraud and to recognize the black sheep that led to this reproach. The following is a try to clear these questions.
Physiological Institute of the Ludwig-Maximilian-University Munich confirmed effectiveness:
"The replacement of laser tubes because of furring through the coolant that had been necessary before, could be avoided after the installation of a physical water treatment device."
Download ReportHotels and instruction companies as well as a lot of conversations with private users confirm the action, although the non-functioning is also often lamented. As in most cases the private users do not know the producer of the device (a lot of times it was said that the product was a cheap one bought in a superstore), we can only draw the conclusion that there are some devices that do not meet the physical conditions to be effective.
But we cannot draw the conclusion that the treatment principle itself is useless and does not work. Unfortunately, this impression is also often given in serious publications, a lot of times without giving any scientific proof or any proof orientated towards the action and that does justice to it. Before the action of the physical water treatment is explained in a plausibility proof, first we have to clarify why water pipes fur up. Therefore we see the lime as target of the physical water treatment.
2. The Lime
How can it be dissolved in the water then?
When water that contains carbon dioxide passes chalky grounds, lime is released and is present in the water as calcium hydrogen carbonate Ca(HCO3)2. This is possible as carbon dioxide CO2 together with water H2O forms carbon acid H2CO3. As everybody knows from the everyday household life, acidic cleaning agents are needed to remove lime deposits. It seems like splitting hairs to underline the difference between dissolved and undissolved lime, but this is exactly where the lack of argumentation in favor of the action of these devices lies.
Primary Deposit Spots
- Pipe bends & branches
- Ending points (faucets)
- Warm water areas (heat exchangers)
Thereupon the following question is raised: why does lime separate anyway?
The dissolved amount of calcium hydrogen carbonate in the drinking water never reaches the saturation limit that if exceeded leads to the separation of the dissolved substance as a crystal.
Why these spots?
The answer is pretty easy: there has to be an energy gradient that leads to the opening of the water cages around the dissolved ions so that they can react with each other. At the same time the so called lime-carbonic acid-balance has to be disturbed, this means that it has to come to a local lack of CO2. Then the elements look for a crystallization point (nucleus) where to start the crystallization. These spots are always located on the walls of the pipes, these represent the solid base on which the crystals can grow.
More and more elements deposit, the lime deposits grow and incrustations, also known as scale, develop. They consist of calcium carbonate mixed with magnesium compounds, gypsum, silicates and iron compounds (therefore the yellow brownish color). These sedimentations favor corrosion and worsen the heat transmission of heating bars and heat exchangers.
The following formula explains the crystallization process:
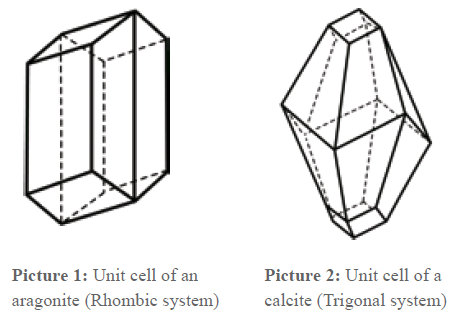
Aragonite or Calcite
When the chemical structure is the same, it depends on thermodynamic circumstances (pressure, temperature) which modification is produced. As shown, in both unit cells, one axis is longer than the others. This means that a crystal grows faster in this direction than in the others. The grow velocity is anisotropic, i.e. dependent on the direction.
That means that crystals that grow undisturbed develop a needle-shaped form. If the grow velocity was the same in all axis directions, globular crystals would develop.
So what do devices do when they show the promised effects?
The answer lies in the physical principles explained in the following sections.
3. Water
To understand the following processes, now some information about water is given. It is way more than what the formula H2O says. The two hydrogen atoms and the oxygen atom form an equilateral triangle and incircle a ~110° angle, as shown in picture 3.
This is the reason for a lot of characteristics that distinguish water from other, similar molecules. Two gases that react with each other form a liquid and not a gas. Because of this angle position water molecules form chains and clusters that cause the fluid state.
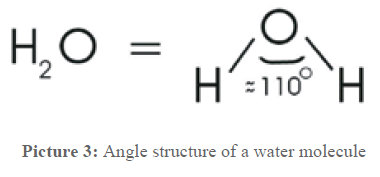
Picture 3: Molecular structure of H2O
This is possibly the reason why water may have a "memory" in which it adopts structures in the chains and clusters that do not change even when the water moves. These chains and clusters are held together by Van de Waals powers or dispersion powers or hydrogen bridges. The bond is based on the attraction of electric dipoles present in molecules with polarized bonds or angled structure.
If the water molecule is put in an electric field, it lines up so that the oxygen shows towards the positive electric side and the hydrogen molecules towards the negative electric side. This fact, together with the molecule form, plays an important role for the dissolving ability of water and for the physical water treatment.
4. Physics and Chemistry
What happens physically and chemically when a physical water treatment device is used?
The processes are described on the basis of a device with an appearance often found and which effects are questioned. Picture 4 represents this device. It is a blackbox from which two cables exit and are wound around the pipe. These cables transmit oscillations to the water that are supposed to "convert" the dissolved lime and render it harmless.
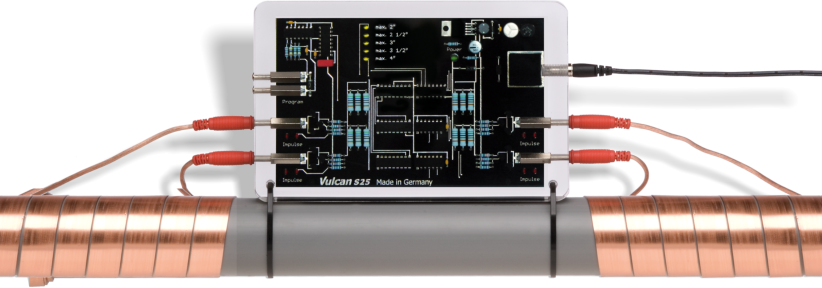
Picture 4: Vulcan treatment windings on the pipe
The Principle of "Influence"
Most doubts begin here: how can an electric field be caused in the water through every pipe material? This is the point of a physical effect called Influence. The winding represents a part of a capacity—it is one capacitor surface, and the other one is the water.
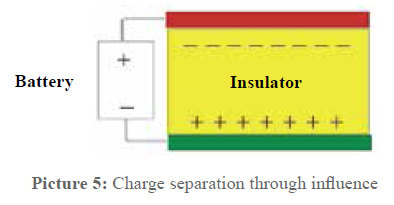
Picture 5: Influence in a capacitor
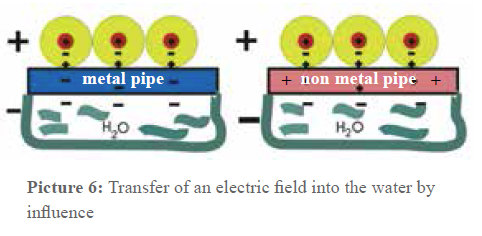
Picture 6: Treatment by Influence
A capacitor is impermeable for direct voltage but not for alternating voltage. This fact is used to introduce electric alternating fields in the pipes. Picture 6 shows that the pipe material does not have any influence on the capacitor effect in the arrangement. If it is a temporal periodic charge transfer, a so-called displacement current is produced between the insulated winding wire and the pipe wall.
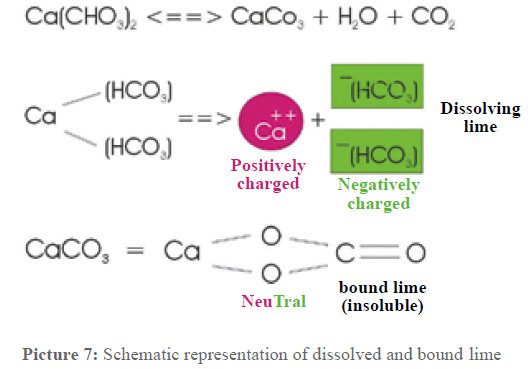
Molecular Dissociation
The dissolved lime—calcium hydrogen carbonate—dissociates in one double positively charged calcium ion and two negatively charged hydrogen carbonate ions. These ions are surrounded by a "water cage."
Picture 7: The Water Cage (Cluster)
The periodically alternating field inside the pipe influences the ions closed in water cages, causing them to move to the beat of the field. This electric oscillation leads to an oscillation of matter (acoustic longitudinal wave). This causes the water cages to disintegrate, freeing the dissolved lime ions to react and form neutral lime crystals in the water that do not react with tap water or existing deposits.
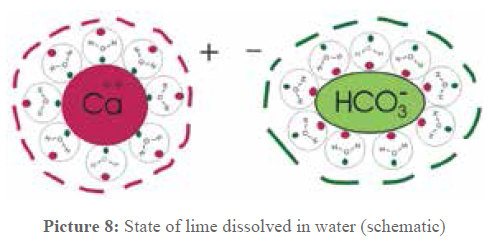
Picture 8: State of lime during treatment
Reaction Velocity
The formation of these molecules occurs between 10 and 20 femto seconds (1 fs = 10⁻¹⁵ second). In the time light travels 6 mm, 1000 molecules can be formed. Therefore, it is very probable that the molecule formation and the formation of nucleus crystals take place in the section treated.
5. Protective Layers and Incrustations
The formation of incrustations is primarily driven by the processes in pipe bends. In these areas, water accelerates, creating a velocity difference between the outer and inner radius. According to the simplified Bernoulli's equation (2), the sum of static and dynamic pressure remains constant:
In faster-flowing water, dynamic pressure increases and static pressure decreases, causing CO2 to escape. This disturbs the lime-carbonic acid balance, setting lime free to find a crystallization point on the pipe walls. Little by little, a layer of lime epitaxially grows, trapping other minerals and eventually clogging the pipe.
The Importance of Lime
Drinking water must maintain a minimum hardness (8.4°d per German decree) because it provides essential calcium and forms a metal carbonate protective layer that prevents corrosion.
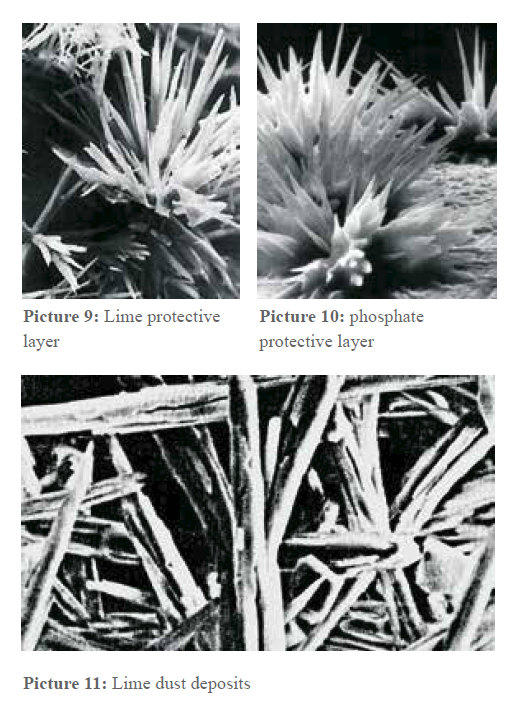
Picture 9 & 10: Microscopic views of protective layers and crystal bundles.
Picture 10 (an electron microscopic image) shows a phosphate protective layer. Desirable at first, these layers become a disadvantage as they serve as ideal crystallization points for further scale growth. However, if the lime is transformed into submicroscopic crystals by Vulcan, they remain in suspension.
The Result:
The lime is washed out of the pipe in form of a fine submicroscopic crystal; crystallization on the walls is no longer possible.
User reports confirm that even in 150-liter water boilers, heating bars remain scale-free, with lime simply depositing as fine dust at the bottom that is easily removed.
6. Removal of Deposits and Protection Against Corrosion
Can these devices remove existing scale? Yes.
Looking back at equation (1), the chemical reaction is reversible. If there is a surplus of carbonic acid, lime is dissolved. For every lime molecule crystallized in the water by Vulcan, a carbonic acid molecule is produced. This "freed" carbonic acid gradually attacks and dissolves existing incrustations on the pipe walls.
The process typically takes between 6 months and 2 years. While the incrustation is removed, the essential carbonate protective layer is maintained, ensuring the pipe remains protected against rust.
Corrosion often occurs due to "ventilation elements" where different oxygen concentrations create potential differences. By removing irregular deposits, Vulcan ensures uniform oxygen contact, eliminating the potential for electrolytic corrosion.
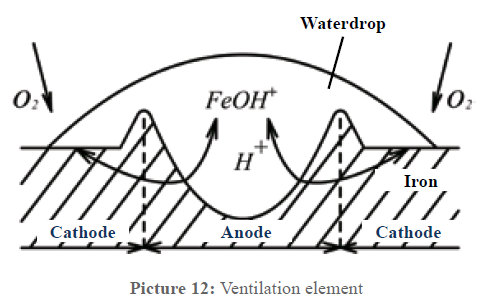
Picture 12: The process of an electrolytic corrosion element.
This is critical for copper pipes, which are susceptible to pitting corrosion at certain pH values. By ensuring a thick, uniform protective layer, Vulcan prevents copper from leaching into the water—a vital health consideration for households with infants.
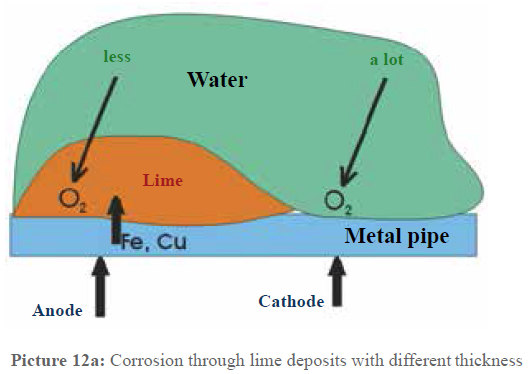
Picture 12a: Uniform thickness of the protective layer.
7. Closing Remarks
The facts show that the effectiveness of physical water treatment is supported by both user experience and physical-chemical proof. However, the electronic demand is high; cheap "superstore" devices often fail to meet the required parameters. Traditional short-term tests often fail to capture these long-term molecular shifts, necessitating new testing procedures for quantitative proof.
Acknowledgements
- Prof. Dr. H. Ungenannt (Magdeburg)
- Mr. K. Matthies (Dipl.-Ing., Berlin)
- Prof. Dr. W. Morgner (Eichenbarleben)
- Christiani Wassertechnik GmbH
Photo Credits
- • Pictures 1 & 2: W. Kleber, Einführung in die Kristallographie
- • Pictures 4, 6, 9 & 11: Christiani Wassertechnik GmbH
- • Picture 10: BMW motorcycle factory Berlin
- • Picture 12: W. Schatt, Einführung in die Werkstoffwissenschaft

